Diffusion:
Diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration.
High Concentration: amount of substance (higher), volume of fluid (low)----many particles
Low Concentration: amount of substance (low), volume of fluid (higher)-----little/few particles
-Size of Particles
- Is a spontaneous process ( no input of energy requires)
- Substances tend to spread from an area where they are more concentrated to an area where they are less concentrated
- 2 or more substance can become evenly distributed (reach equilibrium)
Concentration = amount of substance (solute) / volume of fluid (solvent)
Factors:
-Temperature-Size of Particles
- Is a spontaneous process ( no input of energy requires)
- Substances tend to spread from an area where they are more concentrated to an area where they are less concentrated
- 2 or more substance can become evenly distributed (reach equilibrium)
Concentration = amount of substance (solute) / volume of fluid (solvent)
Concentration Gradient:
The concentration gradient between points A and B is change in concentration between points A and B.
Example:
Sugar molecules diffuses down the concentration gradient from point A to point B
- Particles diffuses down the concentration gradient
Example:
Sugar molecules diffuses down the concentration gradient from point A to point B
- Particles diffuses down the concentration gradient
- The larger the concentration gradient, the faster the rate of diffusion.
Applications of diffusion in Biology
Chemical substances must be able to move from one place to another in order to keep the living organisms alive and growing.
For example, food substances that were absorbed need to:
• move from one cell to another
• move in & out of the cell
• move from one part of the cell to another
| Permeable Membrane | Partially Permeable Membrane |
|---|---|
| Allows all substances to pass through | Allows some substnaces to pass through |
Other examples of diffusion in Biology include:
• Movement of carbon dioxide during photosynthesis
• Movement of oxygen and carbon dioxide in animals
Key Points:
- Diffusion is an important process where substances are moved without use of energy
- It is net movement of particles from a region of higher concentration to a region of lower concentration.
- Thus the movement is down a concentration gradient.
- The movement is random
- The greater the concentration gradient, the faster the rate of diffusion
Osmosis:
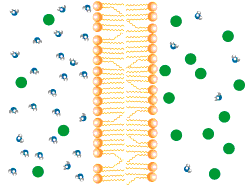
Osmosis is the net movement of water molecules down the concentration gradient through a partially permeable membrane.
Water moves freely through pores in the partially permeable membrane.
• Solute (green) too large to move across the membrane
Differences of Diffusion & Osmosis
- Diffusion: Movement of particles in general
- Can occur both in the presence and absence of a membrane
- Osmosis: Movement of water molecules only
- Water molecules move across a partially permeable membrane
Water Potential
Water Potential is a measure of the tendency of water molecules to move from one area to another.
| Concentration | Water Potential | |
|---|---|---|
Surrounding Area |
WATER (LOW) SUGAR (LOW) | HIGH |
| Within Visking Tubing | WATER (LOW) SUGAR (HIGH) | LOW |
Since Water Potential is a measure of the tendency of water molecules to move from one area to another, we can also define osmosis as:
The net movement of water through a selectively permeable membrane from a region of high water potential to a region of low water potential
Animal Cell:
Animal cells
• Structure is simple
• Cytoplasm is surrounded by a partially
permeable cell membrane.
- If placed in a hypertonic solution (solution has a higher concentration of solutes than the cytoplasm).
- If cell has Lower solute concentration (high water potential) while the surroundings is Higher solute concentration (low water potential), Water leaves the cell by osmosis. The cell loses volume and shrinks (crenates)<----ANIMAL ONLY!
- Water loss only ceases if the concentration of the cytoplasm rises to that of the surrounding solution. Water molecules leaving the cells is the same as water molecules going inside the cell.
- Water leaves the cytoplasm and vacuole by osmosis.
- If placed in a hypotonic solution (solution has a lower concentration of solutes than the cytoplasm).
- If cell has Higher solute concentration (low water potential) while the surroundings is Lower solute concentration (high water potential), Water enters the cell by osmosis. Since the cell membrane cannot reist expansion, the cell eventually bursts (crtolysis)<----ANIMAL ONLY!
Plant Cell:
- Plant cells are structurally more complex.
• They are surrounded by a cellulose cell wall
which is…
– Freely permeable to water
– Not elastic
– Able to resist cell expansion
Each plant cell contain a large central vacuole which…
– contains a solution of salt, sugars and ions
– is bound by a partially permeable membrane
- Water enters the vacuole by osmosis. If placed in a hypotonic solution (solution has a lower concentration of solutes than the cytoplasm) Vacuole swells, pushing the cytoplasm against the cell wall. The inelastic cell resists expansion and the cell become turgid. It can be described in the state of turgor. Young plants (have little woody tissue), rely on turgor for support against wind and gravity.
- Water leaves the cytoplasm and vacuole by osmosis.
If placed in a hypertonic solution (solution has a higher concentration of solutes than the cytoplasm). The cytoplasm and vacuole shrinks, pulling the cell membrane away from the cell wall. The cell is now plasmolysed or is in a state of plasmolysis. The tissue becomes flaccid.
PLASMOLYSIS VS CRENATION:
Plasmolysis:
- shrinking of plant cell cytoplasm, and the cell membrane moves away from cell wall.
Crenation:
- shrinking of animal cell
Isotonic Solution
An isotonic solution has the same concentration of solutes as the cytoplasm.
--> No net movement of water molecules into or out of the cell ( animal & plant)
Therefore, cells neither shrinks nor expands when placed in an isotonic solution.
Water Potential:
- A measure of the tendency of water to move from one place to another.
- A dilute solution has more water molecules per unit volume than a concentrated solution, so it has a higher water potential than a concentrated solution.
- Water always moves from a solution with a higher water potential to a solution with a with a lower one, that is, down a water potential gradient.
It should be noted that the cell wall of plant cells is permeable and allows most substances to pass through. A plant cell behaves differently from an animal cell when placed in solutions with differing water potentials. This difference is due to the presence of a cell wall in plant cells.
What happens to a plant cell when placed in a solution with higher water potential?
- When a plant cell is placed in a solution of higher water potential, the cell sap has a lower water potential than that of the solution outside the living cell.
- By osmosis, water enters the cell through the partially permeable cell surface membrane.
- The cell expands and becomes turgid.
- As water enters the cell, the vacuole increases in size and pushes the protoplasm against the cell wall. The cell does not burst because it is protected by the inelastic cell wall. The turgidity of the cell with water is called turgor. The pressure exerted by the water in the vacuole on the cell wall is the turgor pressure.
What happens to a animal cell when placed in a solution with higher water potential?
- An animal cell will swell and may even burst in a solution of higher water potential than the cytoplasm. This is because unlike plant cells, animal cells do not have a cell wall to protect it.
Turgor plays an important role in maintaining the shape of soft tissues in plants. Most leaves and young stems, especially those of herbaceous and non-woody plants, are able to remain firm and erect because of the turgor pressure within their cells. When there is a high rate of evaporation of water from the cells, they lose their turgidity and the plant wilts.
What happens to a plant cell when placed in a solution with lower water potential?
- When a plant cell is immersed in a solution with lower water potential, the water potential of its cell sap is higher than that of the solution outside the cell.
- By osmosis, water from the vacuole and cytoplasm leave the cell through the partially permeable cell surface membrane.
- The cell decreases in size and becomes flaccid or limp.
- As the cell loses water, the vacuole decreases in size. The cytoplasm shrinks away from the cell wall. The shrinkage of cytoplasm and cell membrane away from the cell wall is called plasmolysis. The cell is said to be plamolysed. A plasmolysed cell can be restored to its original state by placing it in water or in a solution with higher water potential.
What happens to a animal cell when placed in a solution with lower water potential?
- Placing an animal cell in a solution of low water potential will cause it to lose water. The cell shrinks. This process is called crenation. An animal cell will become dehydrated and eventually die when placed in a solution of lower water potential.
Plasmolysis causes tissues to become limp or flaccid. Cells will be killed if they remain plasmolysed for too long.
Soil solution is a thin film of water that surrounds individual soil particles. It usually contains dissolved mineral salts or ions.
1. What is diffusion?

Diffusion is the net movement of molecules moving from a region of higher concentration to a region of lower concentration.
It is one of the transport phenomena that occur in nature, and it results in mixing or mass transport without requiring bulk motion.

More info
2. When will this process stop?
When there is no concentration gradient. The concentration of substances is equal inside and outside. It's called dynamic equilibrium.
Static equilibrium occurs when there is no action taking place.
Dynamic equilibrium occurs when two opposing actions occur at the same rate.
More on dynamic equilibrium
3. Do molecules even stop moving?
In theory, molecules never stop moving as long as the temperature is above an absolute zero. There they reach their minimum motion. When diffusion stops, the concentration of molecules are in equilibrium, but they are still moving.
Full answer
An equilibrium is said to be "dynamic". The reactions going in each direction still take place, they just take place at the same rate, so that the relative amounts of reactants and products do not change.
4. What are the factors that determine the rate of diffusion?
The rate of diffusion is directly proportional to the concentration gradient. The greater the difference in concentration between two areas, the greater the rate of diffusion. Thus, when the gradient is zero, there will be no net diffusion, as diffusion will only occur so long as a concentration gradient exists.
The rate of diffusion is indirectly proportional to resistance. In other words, the greater the resistance to diffusion, the lower the rate of diffusion. Resistance refers to anything that reduces the rate of diffusion. The width of the partitions is a resistance; the wider the partitions, the lower the resistance. And, the membrane is a resistance to the movement of ions and other charged substances in or out of cells.
The rate of diffusion is inversely proportional to distance traveled (also a function of resistance). For example, some typical diffusion rates for water are 10 µm - 0.1 sec; 100 µm -1 sec; and 1 mm - 100 sec. Diffusion is effective over short distances, but is pathetically slow over long distances.
The rate of diffusion is directly proportional to temperature. Temperature increases the rate of molecular movement, therefore, increases the rate of diffusion.
The rate of diffusion is indirectly related to molecular weight (heavier particles move more slowly than lighter, smaller ones). At room temperature, the average velocity of a molecule is fast - about 2 km/sec (=3997 mph!).
Pressure increases the speed of molecules, therefore, increase the rate of diffusion.
Solute particles decrease the free energy of a solvent. Essentially solvent molecules, such as water in a biological system, move from a region of greater mole fraction to a region where it has a lower mole fraction.
In a nutshell:
1. Concentration gradient
2. Resistance
3. Distance travelled
4. Temperature
5. Molecular weight
6. Pressure
2. Resistance
3. Distance travelled
4. Temperature
5. Molecular weight
6. Pressure
Journal:
Write down the key points from today's lesson.

No comments:
Post a Comment
Comments Very Much Appreciated